Services Provided

ISO Cleanroom Certification
ISO Cleanroom Certification Services
We specialize in comprehensive ISO cleanroom certification, ensuring your controlled environments meet the stringent requirements of ISO 14644 standards. Our expert technicians conduct particle count testing, airflow visualization, HEPA filter integrity assessments, and environmental monitoring to validate compliance across ISO Class 1 to Class 9 cleanrooms. Whether you're operating in pharmaceutical, biotech, semiconductor, or medical device sectors, our certification process is designed to support regulatory readiness, product integrity, and operational excellence.

Biological Safety Cabinet Certification
We provide certification services for Biological Safety Cabinets (BSCs) in accordance with NSF/ANSI 49 standards and manufacturer specifications. Our technicians perform rigorous testing including airflow velocity measurements, HEPA filter integrity checks, downflow and inflow validations, and smoke pattern visualization to ensure optimal containment and personnel protection. Whether for Class I, II, or III cabinets, our certification process supports compliance with regulatory requirements and promotes safe laboratory practices. Detailed reports and traceable documentation accompany every service, giving you confidence in your cabinet’s performance.

Fume Hood Certification
Our fume hood certification services ensure that your laboratory ventilation systems meet safety and performance standards, including ANSI/ASHRAE 110 and manufacturer specifications. We conduct thorough face velocity testing, smoke pattern visualization, and containment assessments to verify proper airflow and operator protection. Whether certifying new installations or performing routine compliance checks, our process is designed to support regulatory adherence, minimize exposure risks, and maintain a safe working environment.

Incubator Repair
We offer expert repair services for laboratory incubators, ensuring reliable temperature control, humidity regulation, and CO₂ levels essential for sensitive biological applications. Our technicians diagnose and resolve issues ranging from sensor malfunctions and heating element failures to door seal integrity and control panel errors. Whether servicing dry or humidified incubators, we restore performance to manufacturer specifications while minimizing downtime. Each repair includes functional testing, and detailed service documentation to support compliance and operational continuity in research, clinical, and production environments.

USP Pharmacy Testing
We provide specialized USP pharmacy testing to ensure compliance with critical standards such as USP <797> for sterile compounding and USP <800> for hazardous drug handling. Our services include environmental monitoring, surface sampling, airflow and pressure differential assessments, and cleanroom performance evaluations tailored to pharmacy operations. By validating engineering controls and aseptic practices, we help safeguard patient safety, support regulatory readiness, and maintain operational integrity. Each test is accompanied by detailed documentation and actionable insights to keep your facility aligned with state and federal requirements.
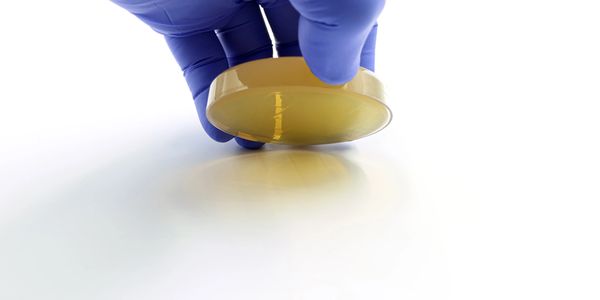
Microbial Sampling
Our microbial sampling services are designed to validate the microbiological integrity of ISO-classified cleanrooms, supporting compliance with ISO 14698 and ISO 14644 standards. We perform active air sampling, passive settle plate monitoring, and surface contact plate testing to detect and quantify viable microbial contaminants. Each sampling protocol is tailored to your cleanroom’s classification level and operational risk profile, ensuring accurate assessment of aseptic conditions. With calibrated equipment, GMP-aligned procedures, and detailed reporting, we help safeguard product quality and maintain regulatory readiness across pharmaceutical, biotech, and compounding environments.
Additional Services
Circle City Cleanroom staff is trained to perform all repairs on any equipment we certify.
Equipment & Room Decontamination
Equipment moves and set-up and disposal
Hepa Filter Replacement and disposal
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.